Navigate
The leading fabless analog ASIC Design Service company
AnSem was acquired by Cyient Ltd, global engineering and technology solutions company, in 2018.
AnSem specializes in designing and delivering state-of-the-art analog,
RF and mixed-signal integrated circuits to customers worldwide.
Wireless personal alarm and nurse call system
AnSem developed an ASIC that integrates a 40kHz UltraSonic receiver for room detection together with an RFID transceiver for room access and wandering prevention. An integrated 640 kHz PLL serves as a clock for the companion RF chip. The ASIC communicates through an SPI interface with this RF Link.

400MHz satellite transceiver for tracking wildlife
Argos is a satellite based communication system that can track the route of a fishing vessel, large migrations of birds and other animals, or can monitor environmental sensor data (atmospheric pressure, sea temperature, animal heart rates) from animals or devices equipped with an Argos tag. These tags are lightweight RF radio systems equipped with multiple sensors (e.g. depth, temperature and light) and they will be mounted on wildlife as to register the trail of the migrating animals. This pattern combined with the sensor's logged measurements will allow scientists to investigate what drives the migration of these animals. What makes Argos unique is the ability to locate the animal anywhere on Earth utilizing the Doppler effect.

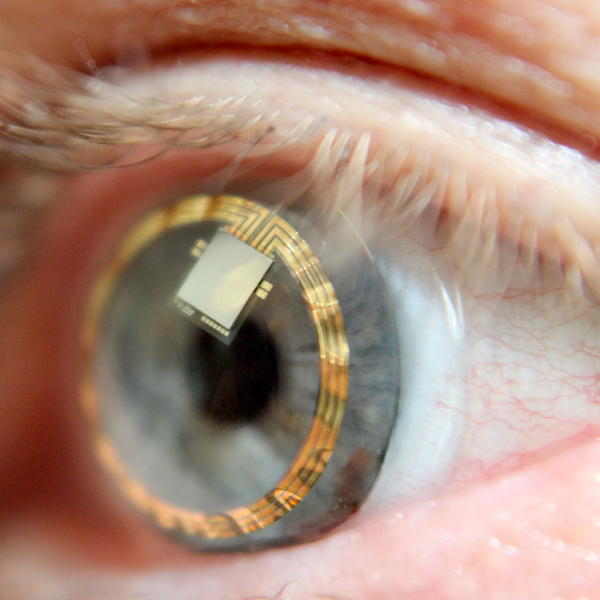
MEMS driver ASIC for a novel contact lens sensor
AnSem developed an ASIC for Sensimed to mount directly within the contact lens. The ASIC digitizes the MEMS sensor reading and transmits the measurements back to the recorder via the same RF link used to power the device using load modulation techniques.
Software defined WiMax radio
AnSem developed for this customer a radio frequency front-end chip containing zero IF, quadrature RX path including wideband LNA and TX path including wideband PA. The device supports a broad, fully programmable RF frequency range between 700MHz and 4GHz, encompassing all licensed commercial spectrum around the globe. A VCO had to be designed with a wide tuning range of 3GHz to 6GHz while complying with tight phase noise characteristics.
Power management for cochlear implants
AnSem developed for Cochlear a highly efficient 24V power management system that generates all internal low and high voltage supplies required in the cochlear implant to perform the high voltage nerve stimulation.The heart of this system is a single inductor multiple output (SIMO) switched inductor regulator. The implementation combines a compact design, requiring a minimum of external components, with advanced switch mode power supplies, LDOs and safety functions.

Your ASIC market
AnSem has profound experience in multiple ASIC markets.
Our ASIC expertise
We have developed a large expertise in different ASIC design areas.
Wireless Data Transmission
State-of-the-art CMOS RF technologies
Wireline Data Transmission
From multi gigabit serial data links to broadband wired communication
Ultra-Low Power
ASICs optimized for low power, single battery applications
High Voltage
Bridging ultra-low power and real-world high voltage applications
Sensors & MEMS Data Aqcuisition
Interfacing sensors for a broad range of applications and markets
Embedded Digital Design
Designing the perfect mix of analog, digital and software
Working at AnSem
We're always looking for talent. Discover what you can do at AnSem.
News & Events
- Your partner in analog, RF and mixed-signal ASIC solutions
- Custom mixed-signal IC design
- Turnkey ASIC supply for leading OEM companies
- Serving the industrial, medical and automotive markets
- Number 1 independent wireless IC design center in Europe
- Bringing innovation on chip
AnSem · Analog, RF and Mixed-signal ASICs
© 1998 – 2024 AnSem. All rights reserved.



